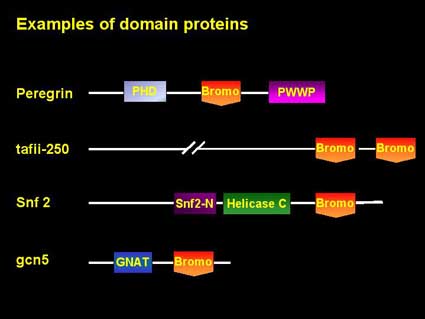
Structure
Structure
A single bromodomain structure is available from HAT
coactivator P/CAF (p300/CBP associated factor). The structure is
an unusual left-handed up-and-down four helix bundle with a left
handed twist. A hydrophobic pocket in the bromodomain creates a
binding site for the acetylated moiety. Intermolecular
interactions are primarily hydrophobic.