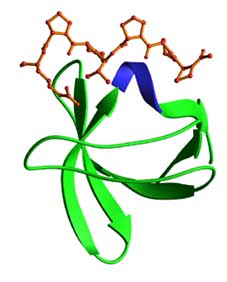
Domain Binding and function
Src-homology 3 (SH3) domains generally bind to Pro-rich peptides that form a left-handed polyPro type II helix, with the minimal consensus Pro-X-X-Pro. Each Pro is usually preceded by an aliphatic residue. Each of these aliphatic-Pro pairs binds to a hydrophobic pocket on the SH3 domain. The detailed requirements of SH3 domain binding to it’s ligand have been examined by numerous approaches including phage display combinatorial peptide chemistry, nuclear magnetic resonance and crystal structure analysis. From this, two classes of SH3 domains have been defined (Class I and Class 2) which recognize RKXXPXXP and PXXPXR motifs respecitvely. The ligand can, in principle, bind in either orientation. Directionality is conferred by the interaction of the Arg or Lys residue with the charged outer face of the SH3 domain while the tandem prolines bind within two hydrophobic pockets of the SH3 domain. An additional non-Pro residue, frequently Arg, can form part of the binding core and contacts the SH3 domain. Such peptides usually bind to the SH3 domain with Kds in the mM range. The binding affinity and specificity can be markedly increased by tertiary interactions involving loops on the SH3 domain. In a few proteins, SH3 domains have been observed to bind in an unconventional non-PXXP manner. In these cases, either an alpha helical element or a tandem tyrosine motif interacts with a site on the SH3 domain that is either distinct or overlapping with the classical PXXP binding cleft.
SH3 domain protein |
Binding partner |
SH3 domain binding site |
| Src tyrosine kinase | p85 subunit of PI 3-kinase |
RPLPVAP Class I N-terminal to C-terminal binding site |
| Crk adaptor protein | C3G guanidine nucleotide exchanger |
PPPALPPKKR Class II C-terminal to N-terminal binding site |
| FYB (FYN binding protein) | SKAP55 Adaptor protein |
RKGDYASY unconventional |
| Pex13p (integral peroxisomal membrane protein) | Pex5p - PTS1 receptor |
WXXQF unconventional |